Error loading player: No playable sources found
3556752
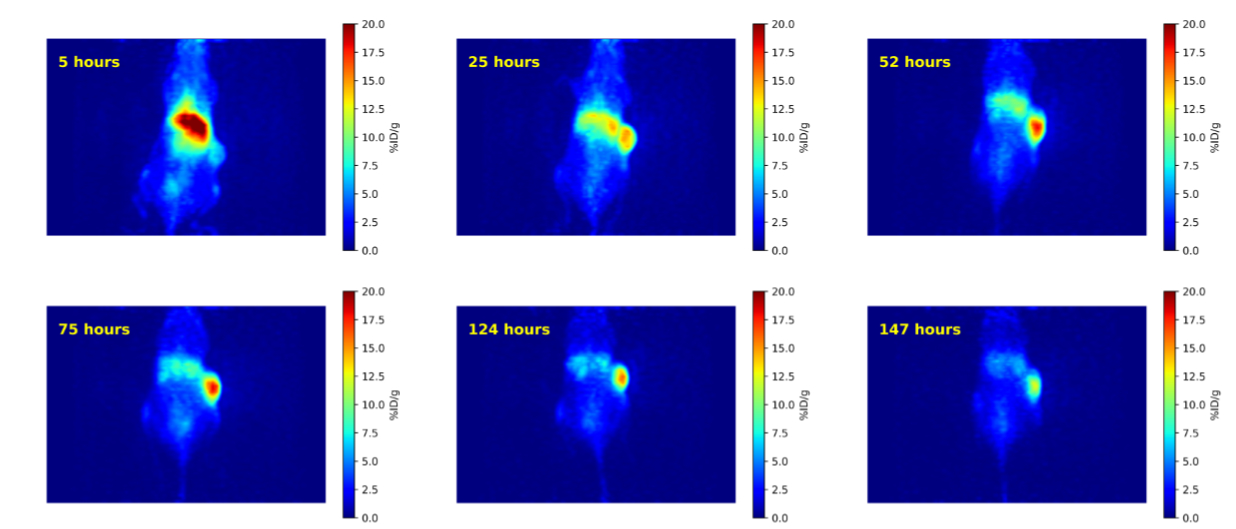
Development of 134Ce/134La radiopharmaceuticals as positron emitting analogues of alpha emitting radionuclides
Date
April 7, 2021
Related Products
Combining structural, spectroscopic, and biochemical tools to explore coordination chemistry across the actinide series
From potential contamination of individuals with radioactive fission products after a nuclear accident to the therapeutic use of radioisotopes for cancer diagnostics and treatment, the biological chemistry of actinides is not only relevant to a number of applied problems, it has also revealed some…
Size and redox control for selective heavy element and inorganic isotope recovery
Over the past decade, select isotopes of the lanthanide and actinide series of elements have emerged as promising therapeutic and/or diagnostic radiometals…
Radiotherapeutics: From Isotope Production to Targeted Radiotherapy:
Division/Committee: [NUCL] Division of Nuclear Chemistry and Technology
Exploring actinium chemistry through the lens of biologically-relevant systems
Targeted alpha therapy (TAT) strategically delivers alpha-emitting radioisotopes to cancerous cells through biological targeting vectors. In particular, actinium-225 (225Ac; t1/2 = 9.92 days) TAT has garnered significant interest with promising clinical studies…