Error loading player: No playable sources found
3529951
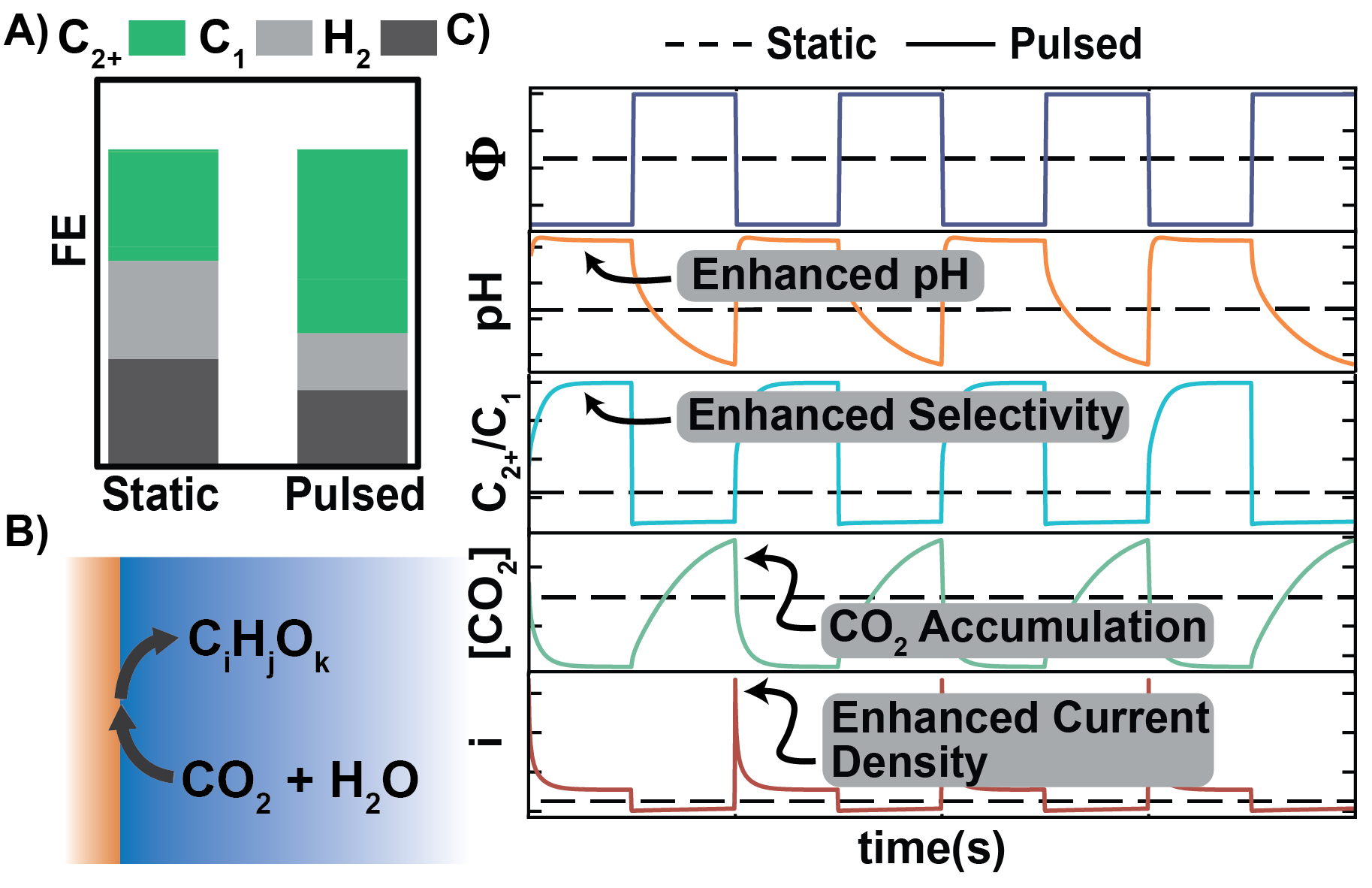
Understanding dynamic mass transport effects in pulsed CO2 electrolysis
Date
April 14, 2021
Related Products
Beyond Tafel analysis for kinetic parameterization in electrochemical synthesis
In recent years, electrochemical synthesis has begun to arise as a viable alternative for the sustainable generation of valuable chemical commodities. Advancement of electrochemical synthesis hinges on the development and characterization of catalysts for these processes…
Continuum modeling to resolve microenvironments in CO2 electrolysis
The electrochemical reduction of carbon dioxide (CO2R) driven by renewably generated electricity (e.g., solar and wind) offers a promising route for reusing the CO2 released during the production of various chemical commodities…
Elucidating the role of nitrile additives in electrochemical CO2 reduction
The organic additive strategy holds promise for enhancing multi-carbon products (C2+) selectivity in electrochemical CO2 reduction (CO2RR), though the roles of these additives, especially nitrile functional groups, are not fully understood…
Electrochemical reduction of CO2: Challenges for materials and system design
The electrochemical reduction of CO2 holds considerable potential as a means for converting CO2 emitted from stationary sources and ultimately from the atmosphere into fuels and chemicals using renewable energy sources (e.g., wind, solar radiation)…