3924587
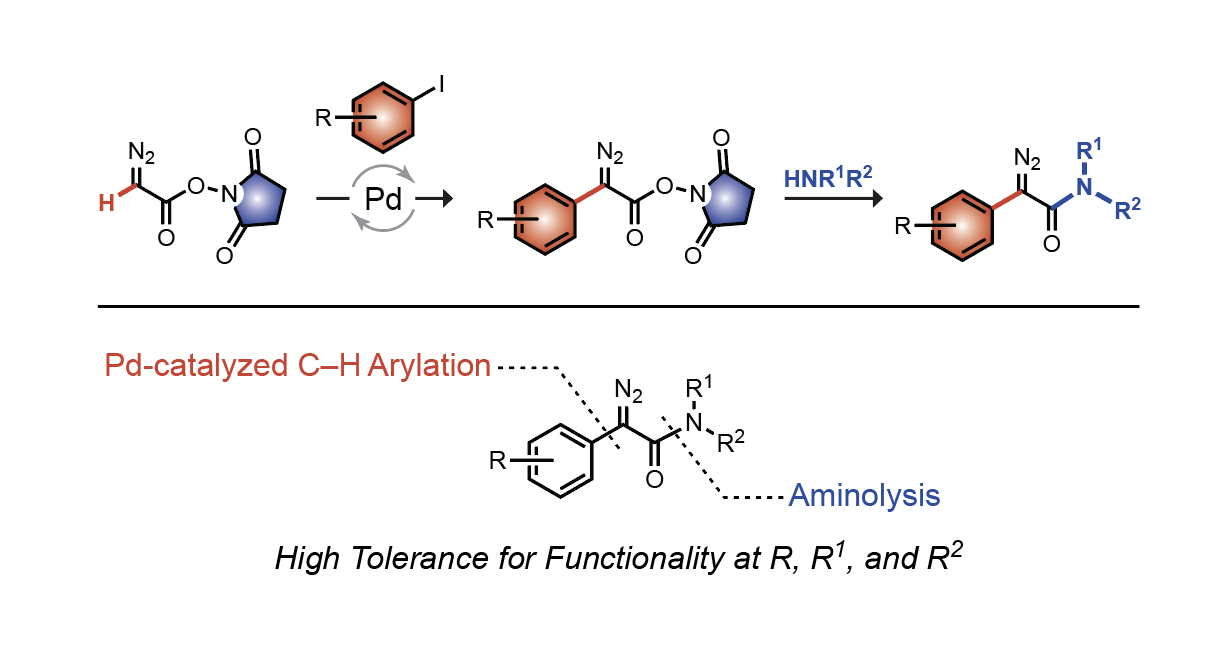
Two-step synthesis of a-aryl a-diazoamides as versatile tools for chemical biology
Date
August 14, 2023
Explore related products in the following collection:
Related Products
Strategies to make chemical catalysis more sustainable
What does sustainability mean in catalysis and how can it be applied in organic synthesis? In this contribution, recent results will be presented which describe two disparate approaches: 1) use of earth abundant metals as replacements for precious metal catalysts and, 2) use of a novel recycling st…
Cascade synthesis of Benzotriazulene with three embedded azulene units and large Stokes shifts
our group successfully constructed a three-pronged star-shaped molecule containing three azulene units by using a previously developed Molecular Strain Engineering (MSE) strategy (_Chem_ 2021, _7_, 2160-2174) based on 8-bromo-1-naphthaldehyde and boronic acid esterified truxene (2) as molecular bui…
Electrochemical fluoroalkylation and amination
In the past few decades, electrochemical reaction has become a powerful tool for radical cross-coupling due to its advantage of green, efficient, mild, and controllable conditions…
Gene therapy without the genes: Intracellular delivery of therapeutic proteins via traceless bioreversible esterification strategy
Following the inception of the first recombinant protein therapeutic—human insulin—into the clinic in 1982, protein-based drugs have flourished, often with greater target specificity and lower toxicity compared to small molecules…