Synthesis and characterization of a solid polymer electrolyte using electrochemical impedance spectroscopy and pulse field gradient NMR diffusion spectroscopy
Related Products
Optimizing OECT performance with polymer blends: Studying volumetric capacitance in poly(3-hexylthiophene) based organic transistors | Poster Board #M34
Organic electrochemical transistors are a developing technology which are made using organic mixed ionic electronic conductors (OMIECs). These devices have applications in biosensing, neuromorphic computing, and other areas…
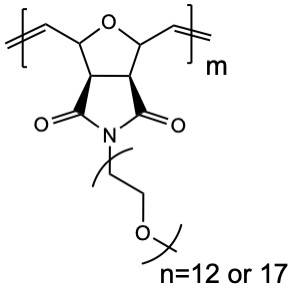
Synthesis and characterization of a solid single-ion copolymer electrolyte for use in lithium-ion batteries | Poster Board #M27
With the increasing use of lithium-ion batteries, the trajectory of the modern world’s energy needs calls for an improvement in their safety and functionality…
Unleashing the potential: Graft-to synthesis of polyoxanorbornene-based single-ion copolymer with tunable glass transition temperature
Lithium-ion batteries are an important electrochemical energy storage technology due to their efficiency and versatility. However, mitigating safety risks such as dendrite formation, electrolyte leakage and flammability is an ongoing challenge…
Synthesis of a ROMP-based electronic conductive macromonomer for copolymerization with an ion conductive macromonomer for improved mixed ionic and electronic conductor properties
Organic mixed ionic-electronic conductors (OMIECs), electric (semi-)conductors with the capacity to transport ionic species, can be used for a wide range of applications including biological sensors or organic electrochemical transistors (OECTs) for use in sensing and circuitry for neuromorphic dev…