3929015
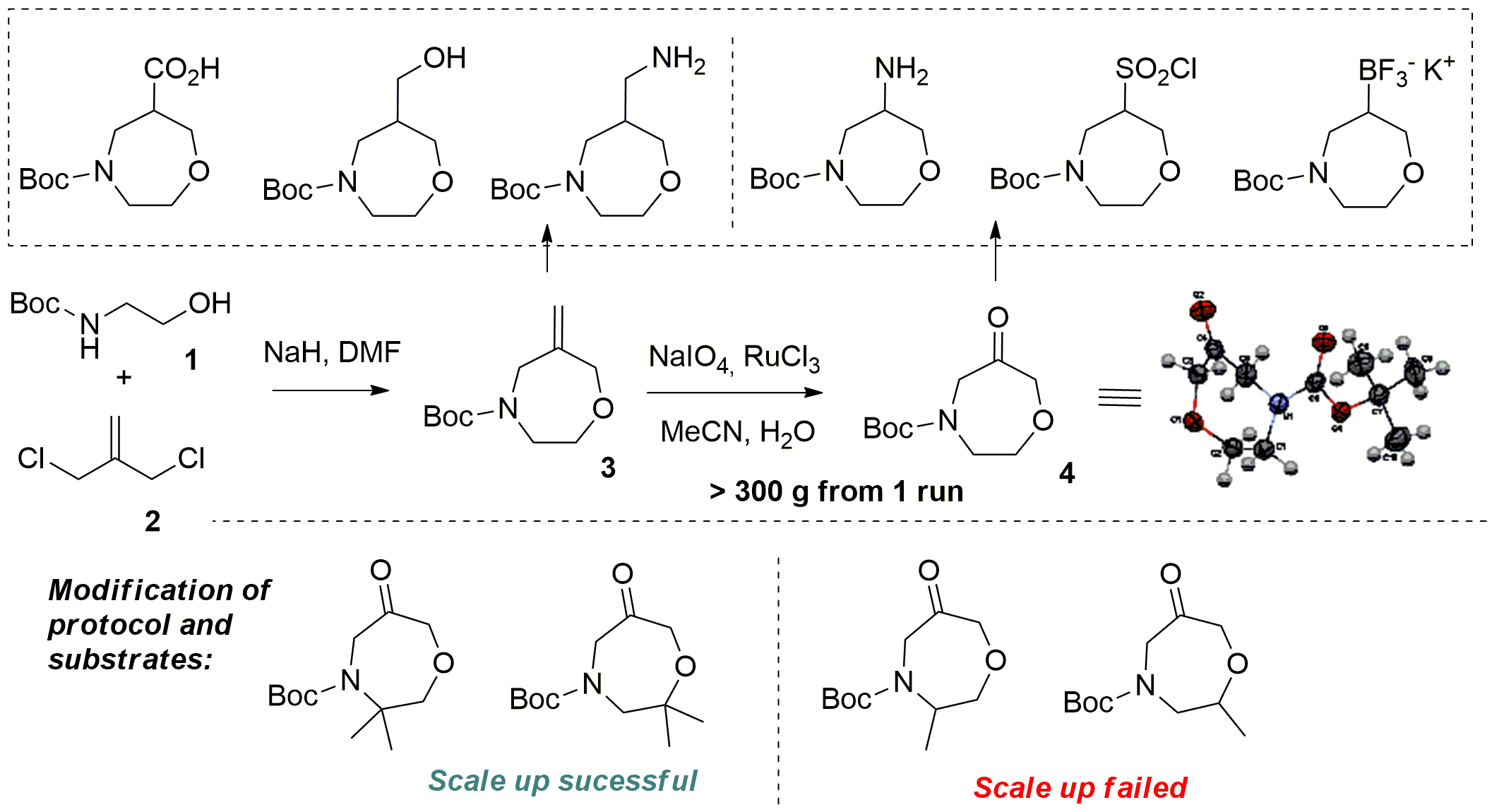
Scale up of 6-methylene-1,4-oxazepane and 6-oxo-1,4-oxazepane derivatives as key intermediates for MedChem relevant building blocks synthesis | Poster Board #3427
Date
August 16, 2023
Explore related products in the following collection:
Related Products
Air-stable efficient nickel catalyst for hydrogenation of organic compounds | Poster Board #3868
A series of composites containing nanoparticles of NiO (from 1 to 10 % by weight per Ni), deposited on the Norit charcoal, was prepared by decomposition of Ni0 complex Ni(cod)2 (cod = _cis_,_cis_-1,5-cyclooctadiene)…
Diversity-oriented large-scale approaches to functionalized thiopyrans | Poster Board #3421
The Prins reaction belongs to the group of venerable transformations in organic chemistry and has been extensively used as a synthetic step in total syntheses of multiple naturally occurring compounds…
(Het)arylacetic acids via Pd-catalyzed carbonylation by CO of corresponding chlorides: Scope and limitations | Poster Board #3367
Our recently made comprehensive analysis of current state of art of the commercial building blocks space clearly showed the lack of (het)arylacetic acids in comparison with (het)aryl ones. Taking into account the importance of these compounds we decided to fill the gap…
Methyl 3-(trifluoromethyl)cyclobut-1-ene-1-carboxylateas key intermediates for MedChem relevant building blocks synthesis | Poster Board #3429
As part of our program to expand MedChem relevant building block chemical space we tested a methyl 3-(trifluoromethyl)cyclobut-1-ene-1-carboxylate 2 as a Michael acceptor in a set of the reactions…