Probing short time diffusion of NISTmAb to predict long-term therapeutic stability of monoclonal antibodies
Related Products
Probing short time diffusion of NISTmAb to predict long-term therapeutic stability of monoclonal antibodies
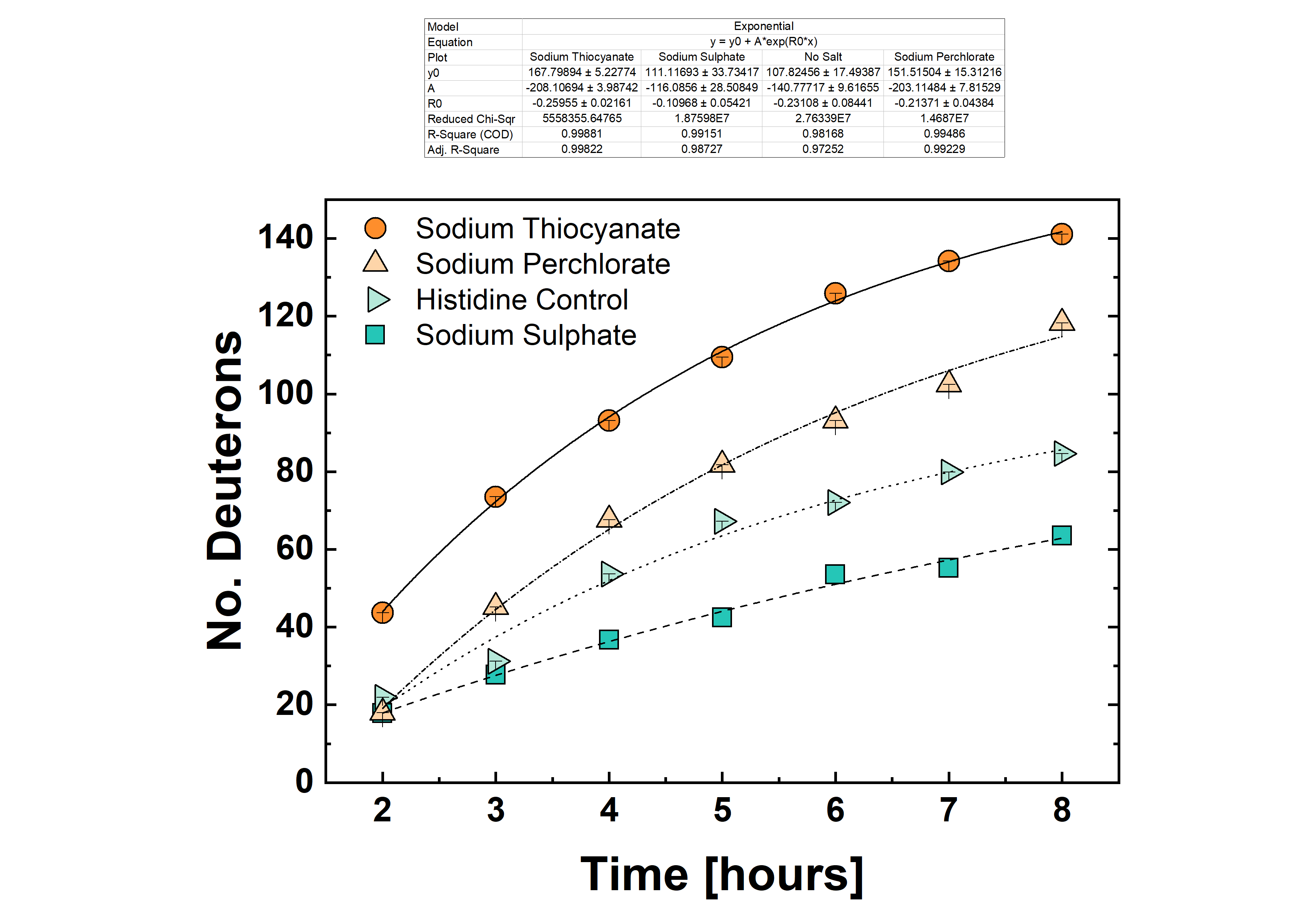
Hydrogen deuterium exchange, (HDX) is of increasing interest for characterization of protein dynamics in solution, at equilibrium state of different conformations…
Graduate Student Symposium: Graduate Student Symposium
: [BIOL] Division of Biological Chemistry
Graduate Student Symposium: Graduate Student Symposium
: [BIOL] Division of Biological Chemistry
Probing short time diffusion of NISTmAb to predict long-term therapeutic stability of monoclonal antibodies
Hydrogen deuterium exchange, (HDX) is of increasing interest for characterization of protein dynamics in solution, at equilibrium state of different conformations…