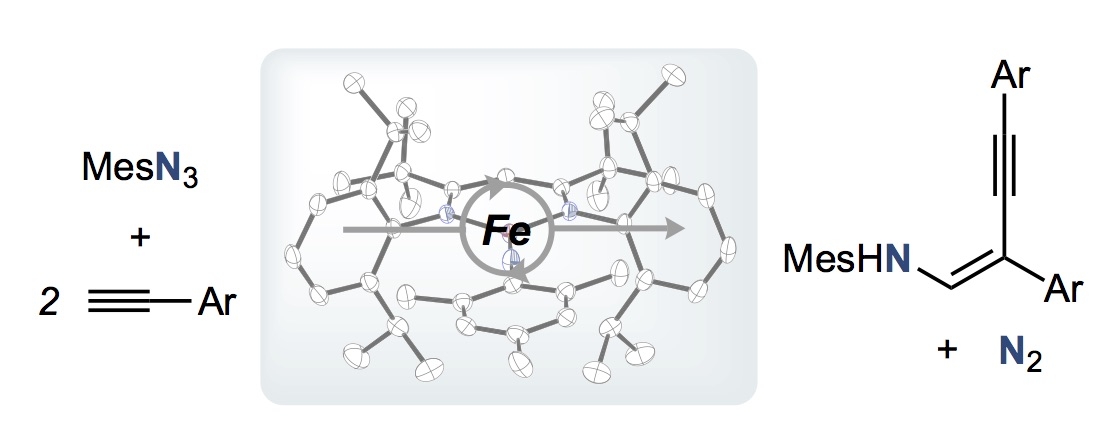
Iron-catalyzed alkyne carboamination via an isolable iron imide complex
Date
March 21, 2022
Related Products
New methods for iron-catalyzed C–N and C–C bond formation with terminal alkynes
First row transition metals present opportunities for the discovery of novel catalytic transformations enabled by their distinct reactivity…
Synthesis and reactivity of manganese porphyrin nitroxido and oximato complexes: Toward manganese-catalyzed C–H amination of hydrocarbon substrates
Activation of C–H bonds in hydrocarbons allows for the use of abundant natural compounds as starting materials…
Investigation of ligand modification in the synthesis and reactivity of iron azametallacyclobutenes
Recent studies in our group discovered a method for generating the first example of a monometallic iron-based azametallacyclobutene. Isolation of this product established a [2+2] cycloaddition mechanism in alkyne carboamination to form β-alkynl enamines from organic azides…
Synthesis and reactivity of manganese porphyrin nitroxido and oximato complexes: Toward manganese-catalyzed C–H amination of hydrocarbon substrates
Activation of C–H bonds in hydrocarbons allows for the use of abundant natural compounds as starting materials…