3733765
Density functional study exploring group 13 complexes for methane activation and conversion | Poster Board #2567
Date
August 21, 2022
Related Products
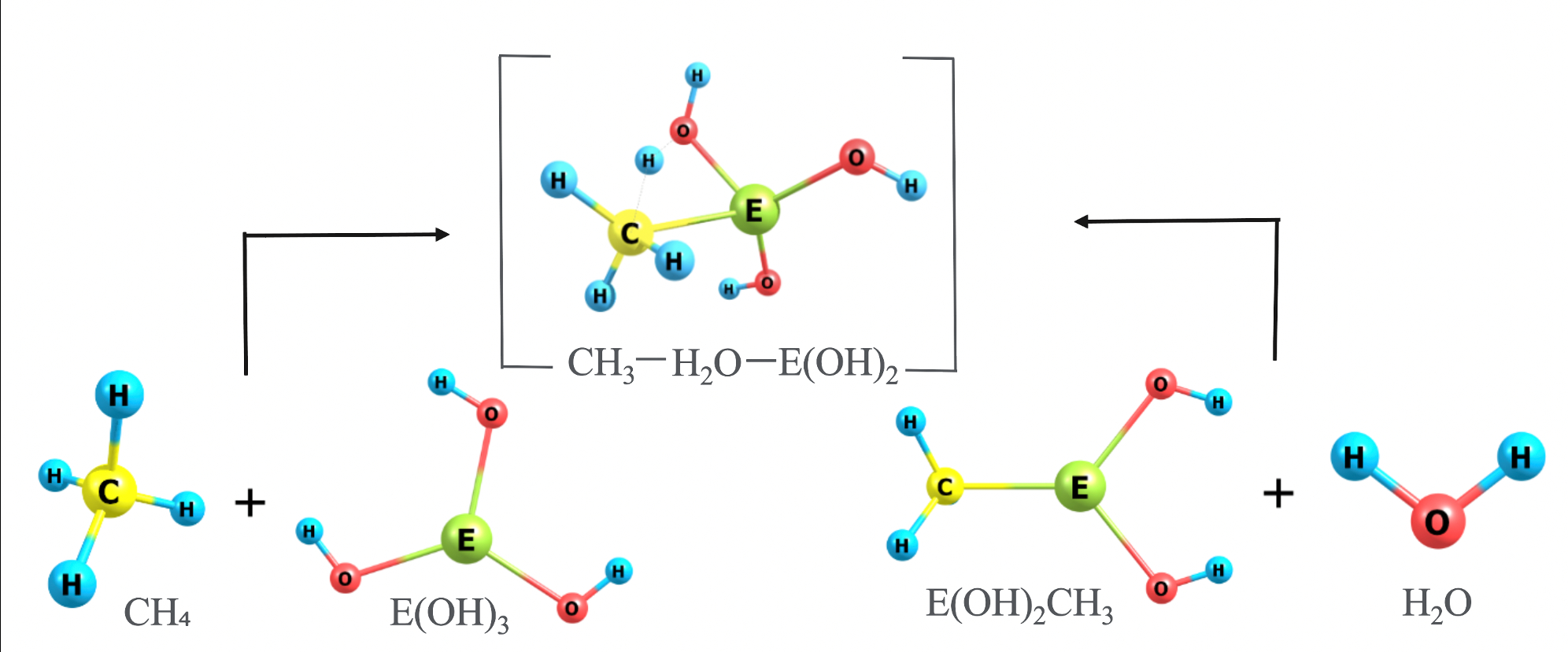
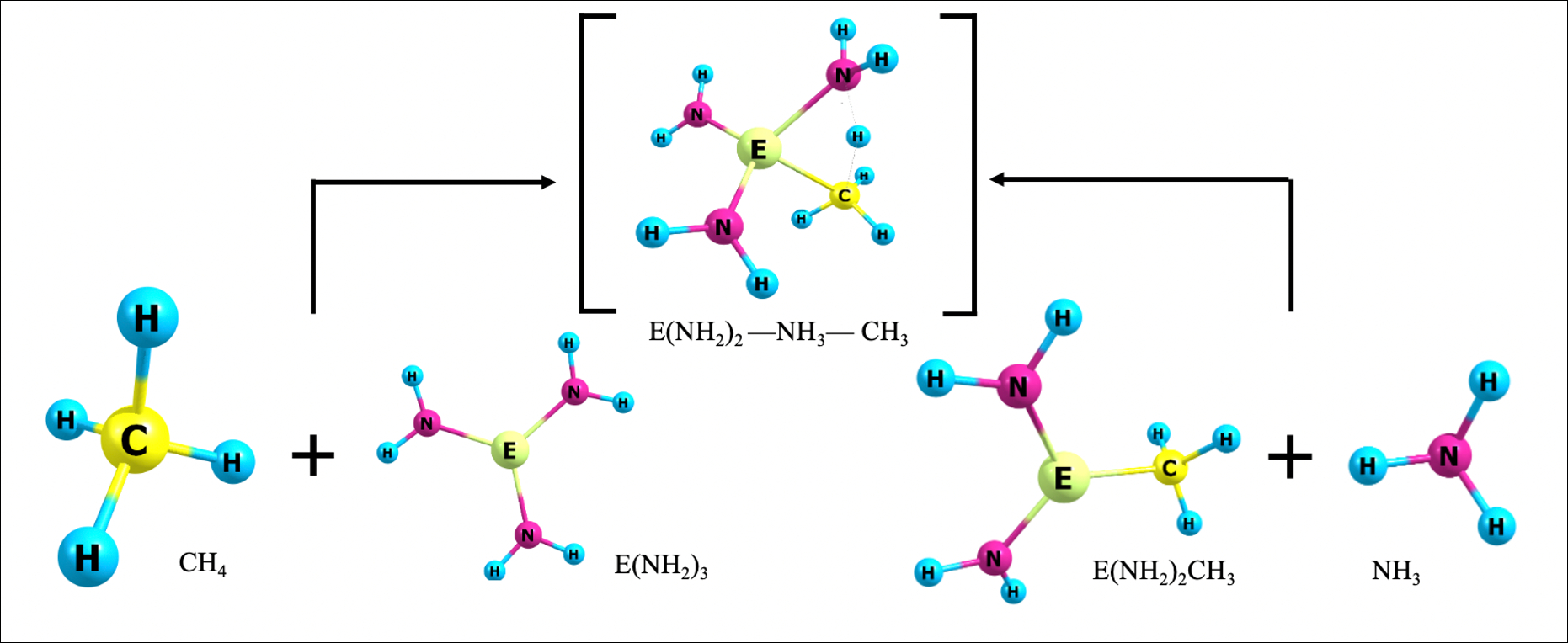
Activation of carbon-hydrogen bonds by complexes involving multiply bonded group 13 elements
Carbon-hydrogen bond activation, especially for light and inert alkanes like methane, is a useful process in catalysis, which can convert these high energy content bonds into more valuable functional groups…
Combined computational and experimental studies of electrocatalytic nitrogen reduction
The electrochemical nitrogen reduction reaction (NRR) is an environmentally and energy-friendly alternative to the Haber-Bosch process for the production of NH3, which currently accounts for ~ 2% of the world’s energy usage…
Chemistry of tetradentate chelate complexes of first row transition metals
Application of several tetradentate chelates to first row transition metals has led to diverse chemistry. Diamide-diimide chelates (dadin-, Medadin-, pdidan-) and hemilabile diamides (bidan-) have shown a varied array of reactivity with small molecules and oxidants…
3d and 4d transition metal nitride mediated N-H bond activation: Ammonia decomposition reaction
The ammonia decomposition reaction is considered as a candidate for the basis of a promising future fuel technology, with ammonia – widely available from fertilizer synthesis – acting as a dihydrogen source/carrier…