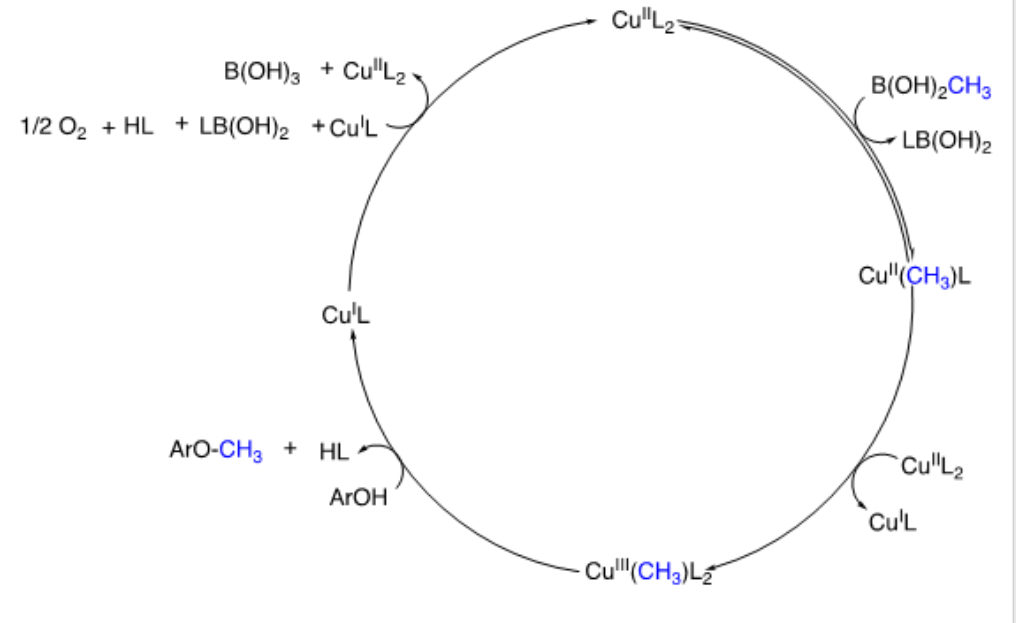
3739237
Cham-Evans-Lam oxidative O-alkylation | Poster Board #2063
Date
August 24, 2022
Related Products
Improving cross-coupling by adaptive dynamic homogenous catalysis
Cross-coupling reactions belong to the most important reactions in organic synthesis in industry and academia. We will discuss, how photochemistry can improve the performance of this reaction class…
Synthesis of DNA-conjucated small molecule catalysts for improved site selectivity | Poster Board #3320
Site selectivity presents a unique challenge when trying to carry out chemical reactions such as functionalization or labeling of molecules in complex systems…
Towards an enzyme mimic through DNA-Palladium conjugation | Poster Board #179
Transition-metal catalyzed reactions in biological contexts are broadly useful. However, these require cytotoxic catalyst concentrations because of the reaction kinetics of bimolecular reactions…