Bifunctional catalysts for cracking of light cycle oil into monoaromatics using a hydrogen donor
Related Products
Theoretical and experimental investigations studying hydrogen donor effect on BTX formation during LCO/LCO model compounds cracking
The polycyclic aromatics present in light cycle oils (LCO) are attempted to convert into mono-aromatic (Benzene, Toluene and Xylene (BTX)) mixture. In the study, n-hexadecane is used as in-situ hydrogen donors, enhancing activity of the hydrogen transfer reactions…
Bifunctional catalysts for cracking of light cycle oil into monoaromatics using a hydrogen donor
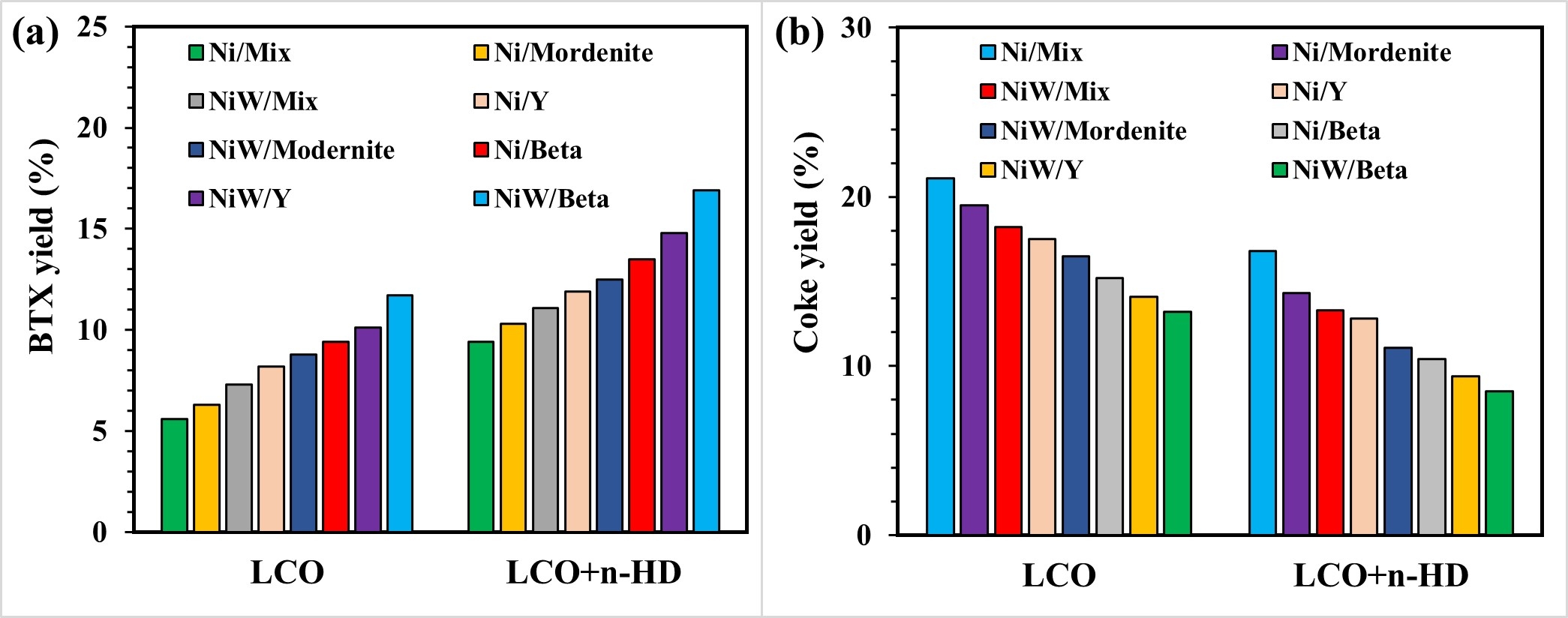
The presence of aromatics (up to 60-80 wt.%) and Sulphur (up to 4 wt.%) in light cycle oil (LCO) makes LCO a poor diesel blend. Catalytic cracking of LCO and its model compounds was performed over monometallic (Ni) and bimetallic (NiW) catalysts supported over different zeolites…
Bifunctional catalysts for cracking of light cycle oil into monoaromatics using a hydrogen donor
The presence of aromatics (up to 60-80 wt.%) and Sulphur (up to 4 wt.%) in light cycle oil (LCO) makes LCO a poor diesel blend. Catalytic cracking of LCO and its model compounds was performed over monometallic (Ni) and bimetallic (NiW) catalysts supported over different zeolites…
Catalytic and Non-Catalytic Upgrading of Heavy Oil and Bio-Crude Oils: Catalytic Upgrading Processes for Heavy Oils
: [ENFL] Division of Energy and Fuels