Error loading player: No playable sources found
3735695
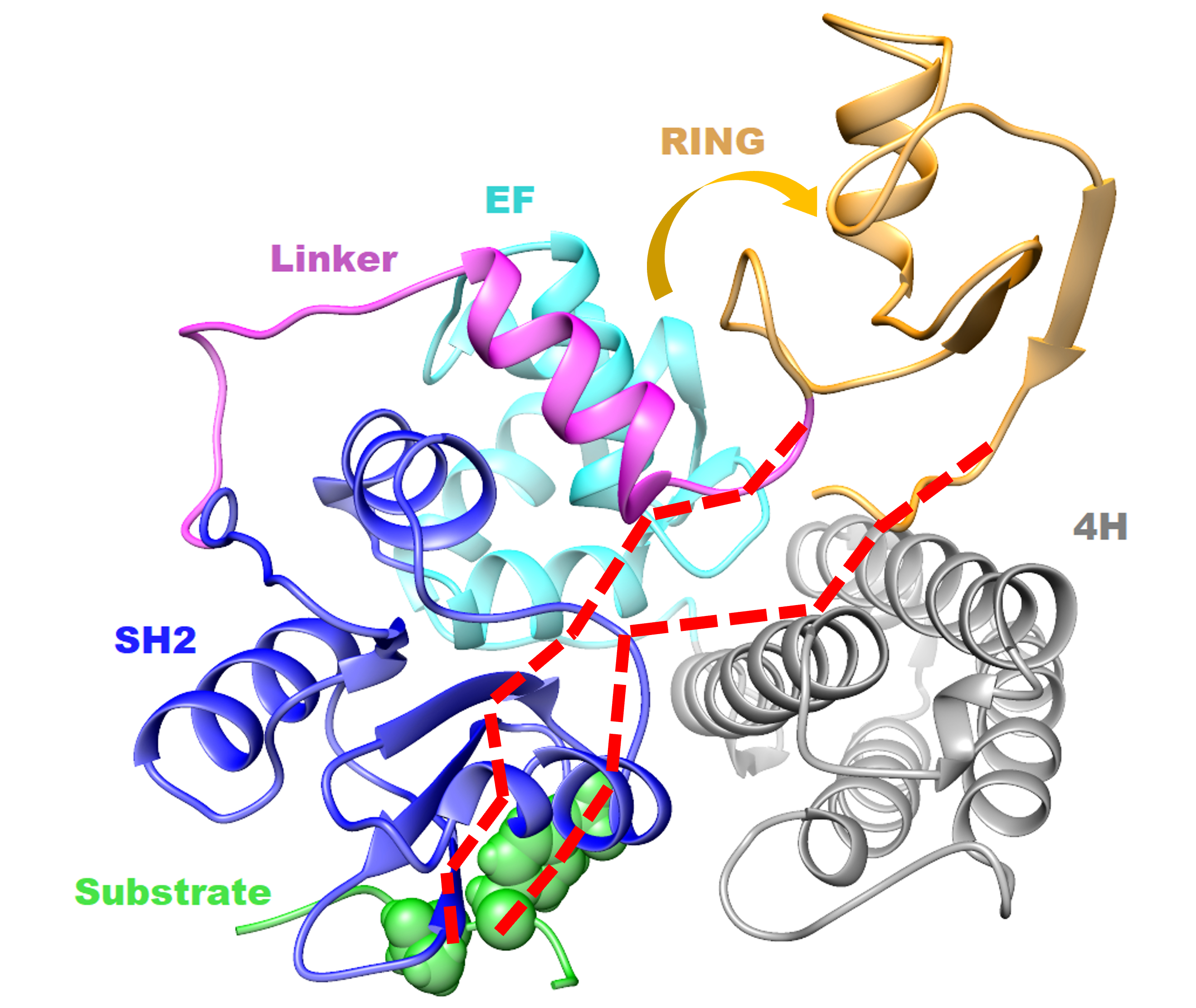
Allosteric signal propagation pathways of unphosphorylated RING-type E3 ubiquitin ligase c-Cbl
Date
August 24, 2022
Related Products
Inverse organic materials design via integration of reinforcement learning with real-time quantum chemistry | Poster Board #439
Generative molecular design strategies have emerged as promising alternatives to library-based high-throughput screening approaches for multi-objective problems in large chemical spaces…
Trans histone pathway of Ubiquitination of nucleosomal H2A by BRCA1/BARD1-UbcH5c Complex | Poster Board #444
The Breast Cancer Associated Protein 1 and its binding partner BRCA1 Associated Ring Domain Protein (BARD1) are a heterodimeric protein that, upon mutation, possess a high risk of breast and ovarian cancer…
Unraveling the dynamics of quantum materials through quantum-classical modeling | Poster Board #440
Quantum materials allow energy, charge, and spin flows to be steered at mesoscopic length scales by means of robust quantum effects, offering exciting technological prospects. It remains, however, unclear how their quantum behaviors emanate from electron-phonon interactions at the microscopic level…
NVIDIA GPU Award:
DIVISION/COMMITTEE: [COMP] Division of Computers in Chemistry