Get the most out of ACS Spring 2025 by signing in with your ACS account.
You are currently browsing the ACS Spring 2025 Event
Preferred
(EDT)
4192529 - Exploring thermoelectric potential: Colloidal synthesis of perovskite-inspired alkali metal chalcogenides | Poster Board #389
10:00 PM - 12:00 AM EDT
Sunday, March 23, 2025 - Monday, March 24, 2025Room: Hall B2/C (San Diego Convention Center)Parent Session
Colloidal Semiconductor Nanocrystals (Including Perovskite Nanocrystals):
Room: Hall B2/C (San Diego Convention Center)
DIVISION/COMMITTEE: [COLL: Division of Colloid and Surface Chemistry]
Credits
0.00 CE
Organizers
- Sohee Jeong, Sungkyunkwan University - Natural Sciences Campus
- Guohua Jia
- Janet Macdonald, Vanderbilt University
- Xuyong Yang
Poster - In-person
COLL: Division of Colloid and Surface Chemistry
Overview
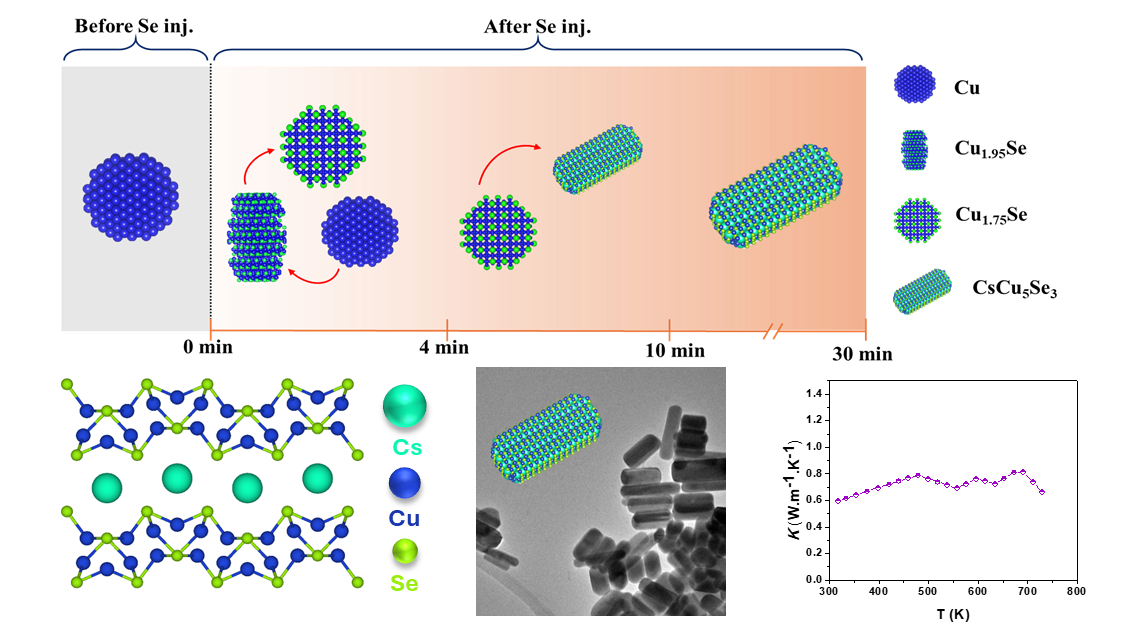
In the last decade, lead halide perovskites have transformed material science due to their outstanding charge mobility, light absorption, and tunable band gaps, all achieved via low-temperature processing. However, their instability and toxicity hinder broader applications. Despite these challenges, perovskites remain promising for future energy technologies, driving the exploration of "perovskite-inspired" materials (PIMs). Recently, attention has turned to alkali metal-based chalcogenides, a new class of semiconducting inorganic compounds, as potential candidates for energy materials. Ternary alkali-metal dichalcogenides {AMeE (A = Li, Na, K, Rb, Cs; Me= Metals, E = S, Se, Te)} have emerged as promising for energy conversion and storage applications.While high-temperature solid-state synthesis often provides limited phase control, wet chemical synthesis offers an alternative by producing uniform nanoscale particles with improved phase precision. Building on cesium copper-based chalcogenide studies, we synthesized nanoscale cesium copper selenide (CsCu5Se3) with precise control over size, morphology, and phase. Key reaction variables, such as precursor reactivity, ligands, reaction temperature, and time, were optimized, demonstrating the flexibility of wet chemical synthesis. An ex-situ mechanistic investigation revealed that nanocrystal formation is driven by the dissolution of binary Cu2-xSe, followed by the incorporation of Cs+ to form ternary CsCu5Se3.
The study further shows that variations in the alkyl chain length of amines influence nanocrystal size, shape, and phase formation. The structural, electronic, and thermoelectric properties were experimentally evaluated and corroborated by computational analysis. Results demonstrated ultralow thermal conductivity of 0.6 W.m-1.K-1 and a promising thermoelectric figure of merit (ZT) of 0.3 at 720 K, highlighting its potential for energy applications. The detailed mechanistic insights from this study offer significant advancements for developing functional materials in the alkali metal chalcogenide family, with broad applicability in energy conversion and storage systems.
The study further shows that variations in the alkyl chain length of amines influence nanocrystal size, shape, and phase formation. The structural, electronic, and thermoelectric properties were experimentally evaluated and corroborated by computational analysis. Results demonstrated ultralow thermal conductivity of 0.6 W.m-1.K-1 and a promising thermoelectric figure of merit (ZT) of 0.3 at 720 K, highlighting its potential for energy applications. The detailed mechanistic insights from this study offer significant advancements for developing functional materials in the alkali metal chalcogenide family, with broad applicability in energy conversion and storage systems.