Get the most out of ACS Spring 2025 by signing in with your ACS account.
You are currently browsing the ACS Spring 2025 Event
Preferred
(EDT)
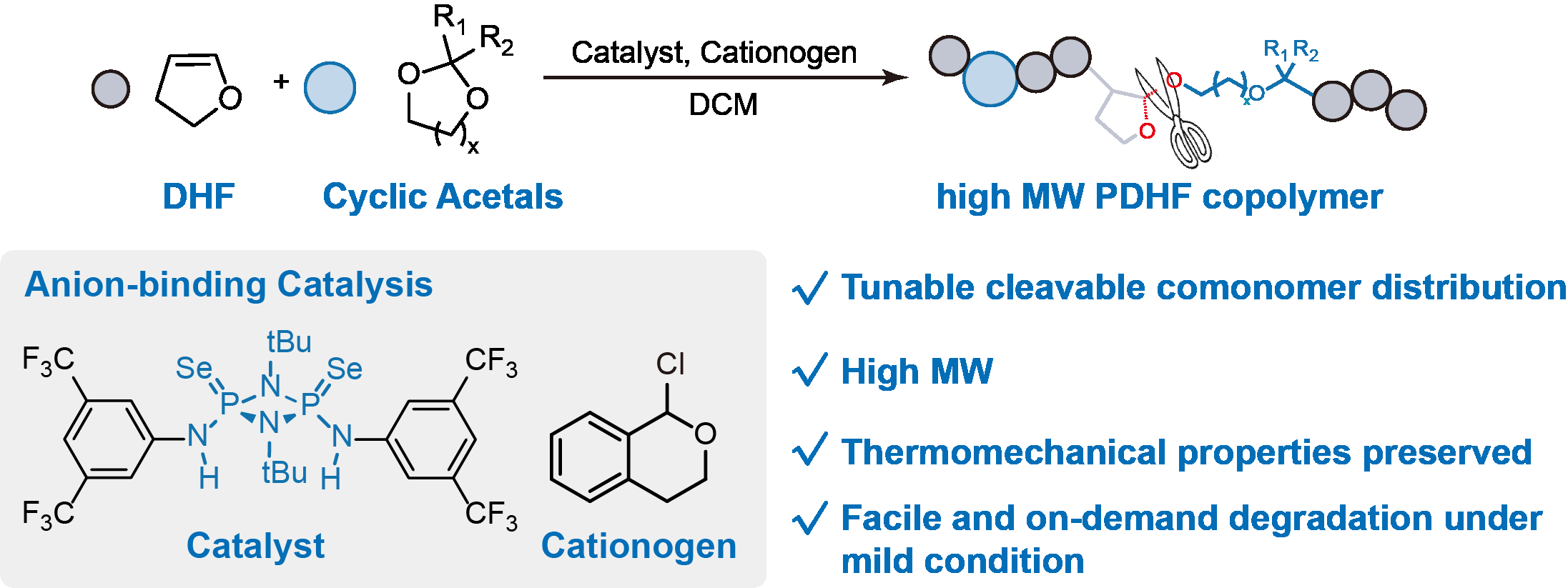
4182985 - On-demand, readily degradable Poly-2,3-dihydrofuran enabled by anion-binding catalytic copolymerization
11:05 PM - 11:15 PM EDT
Tuesday, March 25, 2025Room: Digital Session (Digital Meeting)Parent Session
Virtual Graduate Students Symposium in Asia-Pacific Region on Polymer Chemistry:
Room: Digital Session (Digital Meeting)
DIVISION/COMMITTEE: [POLY: Division of Polymer Chemistry] [PRES: Presidential Event]
Credits
0.00 CE
Organizer, Presiders
Presiders
- Jia Tang, The Dow Chemical Company
- Yanli Feng
Student
POLY: Division of Polymer Chemistry
PRES: Presidential Event
Oral - Digital
Overview
Copolymerization with cleavable comonomers is a versatile approach to generate vinyl polymer with viable end-of-life option such as biodegradability. Nevertheless, such strategy is ineffective in producing readily degradable 2, 3-dihydrofuran (DHF) copolymer with high-molecular-weight (>200 kDa). The latter is a strong and biorenewable thermoplastic that eluded efficient cationic copolymerization synthesis. Here, we show that an anion-binding catalyst seleno-cyclodiphosph(V)azanes enable the efficient cationic copolymerization with cyclic acetals by reversibly activate both different dormant species to achieve both high living chain-end retention and high-molecular-weight. This method leads to incorporating low density of individual in-chain acetal sequences in PDHF chains with high-molecular-weight (up to 314 kDa), imparting on-demand hydrolytic degradability while without sacrificing the thermomechanical, optical and barrier properties of the native material. This methodology can be readily adapted to traditional cationic polymerization to furnish facile degradable polymers with tunable performances for various applications and environmental sustainability.
Presenter
Co-Authors






